Ever wondered why some refractory products endure extreme heat while others fail? The answer lies in their refractory products composition—a strategic mix of materials, additives, and impurities defining thermal and chemical performance. This article deciphers the roles of Al₂O₃ in high-alumina bricks, MgO in magnesia-carbon linings, and SiC in non-oxide composites for steelmaking, glass, and reactors. It covers acid-resistant silica, neutral alumina-silicate, and basic magnesia solutions where minor impurities like iron oxides decide success. Learn how composition mastery transforms raw elements into durable materials surviving 1,500°C while resisting corrosion and stress.
- Understanding the building blocks of refractory materials
- The fundamental elements: main components, additives, and impurities
- A systematic classification of refractory compositions
- Beyond The Brick: Composition Of Specialized Refractory Forms
- How chemical composition translates to performance
- Choosing the Right Composition for Optimal Refractory Solutions
Understanding the building blocks of refractory materials
The performance of refractory products hinges on their chemical composition—a precise equilibrium where each element plays a critical role. Just as a single weak link can break a chain, even minor imbalances in components like SiO₂ or MgO can compromise structural integrity, corrosion resistance, and thermal stability. A 2% deviation in MgO content for magnesia-carbon bricks in steelmaking, for instance, can cut service life by 40%.
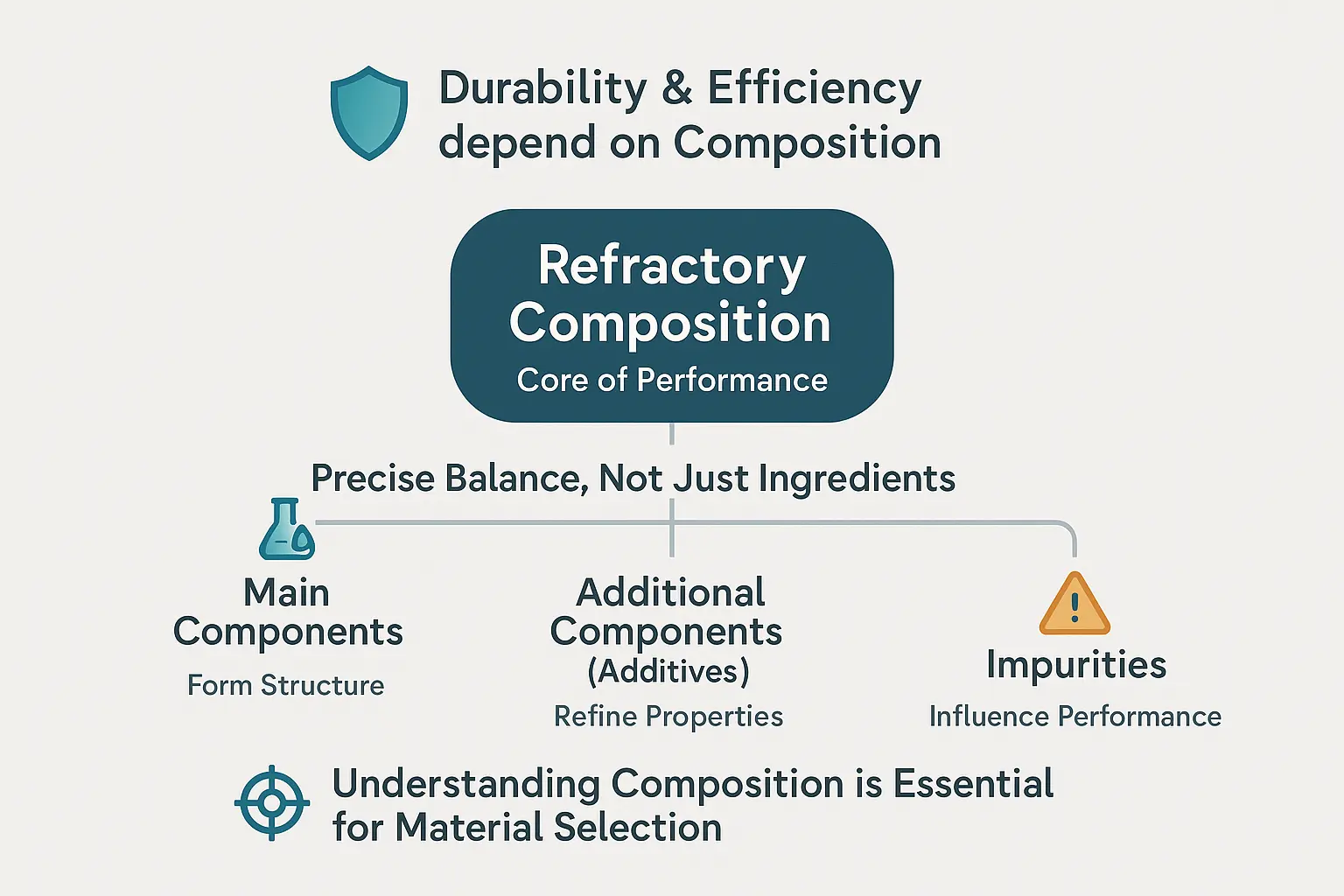
Three core elements define refractory behavior: primary components (like Al₂O₃ or ZrO₂) form the material’s backbone. Additives (mineralizers, stabilizers) refine properties, while unwanted impurities act as silent saboteurs, lowering melting points and accelerating degradation. The Al₂O₃-to-SiO₂ ratio in aluminosilicates, for example, dictates microstructure—higher Al₂O₃ content (≥48%) boosts refractoriness but risks brittleness. Even trace impurities like Fe₂O₃ in silica bricks can trigger phase changes under thermal stress.
This article explores how chemical composition shapes performance. From acidic silica-based bricks (≥93% SiO₂) in glass furnaces to basic magnesia-carbon composites (≥70% MgO) for steel ladles, we’ll decode classification systems and reveal why 70% of global refractory demand in steelmaking depends on oxide ratios. Discover how Cr₂O₃ in magnesia-chrome bricks boosts slag resistance via spinel phases, or why ZrO₂-based materials excel in cement kilns through low thermal conductivity and chemical inertness. The interplay between porosity, composition, and thermal shock resistance highlights how tailored chemistry transforms ordinary materials into high-performance solutions for glass, cement, and metal industries.
The fundamental elements: main components, additives, and impurities
Refractory materials owe their extreme-temperature resilience to precise chemical compositions. Understanding their three core elements—principal components, additives, and impurities—reveals how engineers balance durability with tailored performance.
Principal components: the foundation of performance
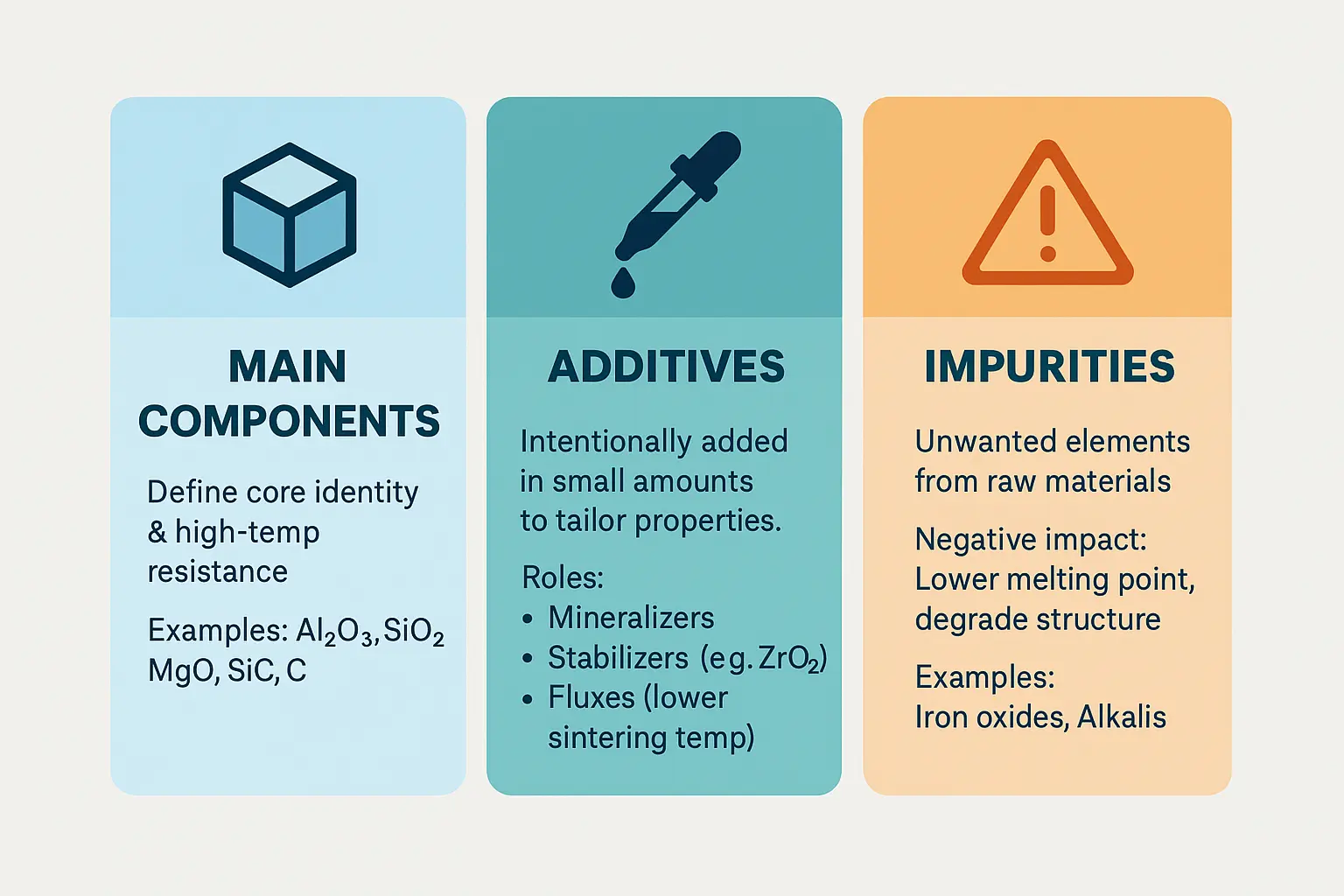
Refractories derive their core properties from principal components, which constitute 50-100% of the material. These high-melting-point substances form the structural backbone:
- Oxides: Al₂O₃ (alumina), SiO₂ (silica), MgO (magnesia), CaO (lime), ZrO₂ (zirconia)
- Non-oxides: SiC (silicon carbide), C (carbon)
For instance, silica bricks (≥93% SiO₂) maintain shape at 1,690°C, while magnesia bricks (>85% MgO) resist basic slags in steelmaking. Silicon carbide enhances thermal conductivity in glass furnaces.
Additional components: tailoring properties with additives
Engineers add 0.1-10% additives to fine-tune characteristics. These intentional inclusions include:
- Mineralizers: TiO₂ accelerates spinel phase formation in alumina-magnesia bricks, improving densification
- Inhibitors: MgCO₃ stabilizes β-Al₂O₃ in corundum castables, enhancing thermal shock resistance
- Fluxes: CaCO₃ lowers sintering temperatures but risks increased porosity
Such additives enable MXS Refractories to customize solutions for glass, steel, and cement industries while maintaining structural integrity.
Impurities: the unavoidable influencing factor
Unwanted elements from raw materials—like Fe₂O₃ (iron oxide) and K₂O (potassium oxide)—create hidden vulnerabilities. Even trace amounts (<1%) can:
- Lower melting points by forming eutectic phases
- Generate liquid phases at operational temperatures
- Accelerate chemical attack from corrosive slags
For example, Na₂O in silica bricks initiates liquid phase formation at 1,000°C—far below silica’s 1,713°C melting point. Contaminants in key raw materials demand rigorous quality control to prevent premature failure.
- Principal Components: Define the material’s core identity and high-temperature resistance (e.g., Al₂O₃, MgO, SiC)
- Additives: Intentionally added in small amounts to modify properties (e.g., stabilizers, fluxes)
- Impurities: Unwanted elements from raw materials that degrade performance (e.g., iron oxides, alkalis)
This chemical triad determines whether refractories endure 1,700°C blast furnaces or succumb to premature spalling. MXS Refractories’ expertise lies in optimizing this delicate balance for specific industrial environments.
A systematic classification of refractory compositions
Refractory materials form the backbone of high-temperature industrial applications, with their chemical composition directly determining performance characteristics. Understanding their classification based on chemical nature reveals critical insights into material behavior under extreme conditions. These materials must withstand thermal stress and chemical interactions with slags, making compositional analysis essential for glass, steel, and cement production where corrosion resistance defines operational longevity.
Oxide-based refractories: the most common families
Oxide-based refractories dominate industrial applications due à leur prévisibilité chimique et leur rentabilité. Leur catégorisation suit des seuils spécifiques de teneur en oxydes :
- Briques siliceuses contiennent plus de 93 % de SiO2, créant des matériaux hautement acides idéaux pour les fours à verre où la résistance aux chocs thermiques est cruciale. Ces briques démontrent leur efficacité dans des environnements où la température varie rapidement entre 1400°C et 1600°C
- Produits aluminosilicatés incluent des briques d’argile réfractaire (30-48 % Al2O3) et des réfractaires à haute teneur en alumine (>48 % Al2O3), passant d’un comportement acide à neutre. Les variantes corindon-mullite montrent une résistance au fluage exceptionnelle à 1600°C grâce à leur structure cristalline organisée
- Solutions à base de magnésie démontrent des caractéristiques basiques grâce à une teneur supérieure à 85 % de MgO dans les briques de magnésie, tandis que les variantes magnésie-chrome combinent plus de 48 % de MgO avec plus de 8 % de Cr2O3 pour une résistance améliorée contre la corrosion dans les environnements dynamiques de sidérurgie. Ces matériaux forment des phases spinelles complexes lorsqu’ils interagissent avec les scories, limitant la pénétration des impuretés
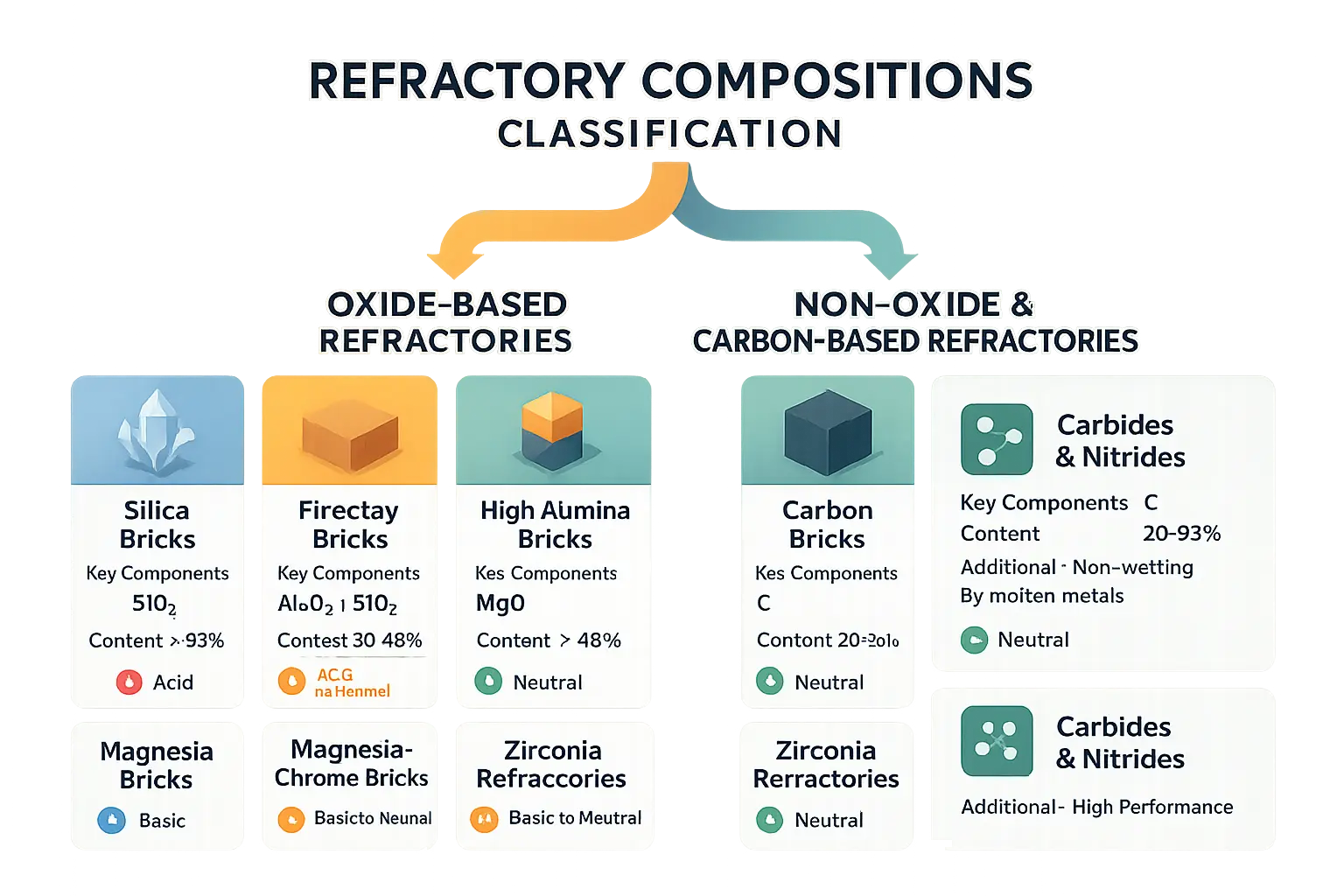
| Refractory Family | Key Chemical Components | Typical Content (%) | Chemical Nature |
|---|---|---|---|
| Silica Bricks | SiO₂ | > 93% | Acid |
| Fireclay Bricks | Al₂O₃, SiO₂ | 30-48% Al₂O₃ | Acid to Neutral |
| High-Alumina Bricks | Al₂O₃ | > 48% | Neutral |
| Magnesia Bricks | MgO | > 85% | Basic |
| Magnesia-Chrome Bricks | MgO, Cr₂O₃ | > 48% MgO, > 8% Cr₂O₃ | Basic to Neutral |
| Carbon Bricks | C | 20-90% | Neutral |
| Zirconia Refractories | ZrO₂, stabilizers | Variable | Neutral |
Les solutions oxydes avancées comme la zircone (ZrO₂) nécessitent des stabilisateurs tels que CaO ou MgO pour maintenir la structure cristalline à haute température. Ces matériaux démontrent une résistance aux transformations de phase cruciale pour les environnements à chocs thermiques. La zircone stabilisée à la chaux maintient sa phase cubique jusqu’à 2400°C, tandis que les variantes stabilisées à la magnésie offrent une meilleure résistance à la corrosion dans les applications métallurgiques grâce à une matrice plus dense.
Non-oxide and carbon-based refractories
Pour des conditions extrêmes où les matériaux oxydes atteignent leurs limites, les réfractaires non-oxydes offrent des avantages distinctifs. Leur structure moléculaire permet une conductivité thermique exceptionnelle et une non-mouillabilité par les métaux fondus, empêchant la pénétration structurelle par action capillaire.
- Matériaux à base de carbone utilisent 20 à 90 % de graphite ou de coke, créant des surfaces neutres résistant à la pénétration métallique. Le ratio graphite/cok influence directement la conductivité thermique, avec un mélange 70/30 atteignant 18-22 W/m·K tout en maintenant une résistance mécanique supérieure à 35 MPa
- Carbure de silicium (SiC) combine une conductivité thermique élevée avec une résistance à l’oxydation jusqu’à 1600°C, rendue possible par des liaisons covalentes fortes et une structure cristalline hexagonale. Ces propriétés en font un choix prioritaire pour les échangeurs de chaleur et les équipements d’industries chimiques
- Céramiques sialon (à base de Si₃N₄) démontrent une résistance supérieure au fluage à des températures dépassant 1400°C grâce aux avantages de la phase β-sialon, offrant 40 % de résistance thermique en plus par rapport au nitrure de silicium conventionnel
Ces matériaux suivent des schémas de performance où une teneur plus élevée en carbone améliore la résistance aux chocs thermiques mais réduit la résistance mécanique, tandis que les combinaisons carbure-nitrure optimisent à la fois l’intégrité structurelle et la stabilité thermique. L’expertise de MXS Refractories dans ces compositions spécialisées permet des solutions adaptées là où les matériaux oxydes conventionnels échouent. Par exemple, les composites à base de carbure de silicium avec ajout de zircone améliorent la résistance à l’érosion de 60 % dans les revêtements de fours à cyclone grâce à des mécanismes de déviation des fissures.
Beyond The Brick: Composition Of Specialized Refractory Forms
The makeup of refractory coatings and mortars
Refractory coatings and mortars are engineered not just for thermal resistance but for application precision. Their composition hinges on specialized binders like calcium aluminate cements or phosphates, ensuring adhesion and structural integrity. Fine fillers—often silica or alumina powders—fill micro-gaps, creating a protective barrier. Unlike traditional bricks, these materials require ultra-fine granulometry to ensure smooth application and bonding, critical for high-stress environments like glass or steel manufacturing.
Composition of advanced refractory composites and monolithics
Advanced composites and monolithic refractories redefine performance in extreme conditions. Their core lies in a ceramic matrix, such as alumina or silicon carbide (SiC), paired with reinforcing agents like ceramic fibers or carbon particles. These elements combat mechanical stress, preventing catastrophic failure. For monolithics like castables, the blend of aggregates, binders, et al. is optimized for flow and setting. Monolithic refractories exemplify adaptability, balancing chemical stability with workability.
- Specialized Binders: Calcium aluminate cements and phosphates ensure cohesion in coatings and monolithics, adapting to thermal cycling.
- Reinforcing Agents: Steel or ceramic fibers enhance toughness, delaying crack propagation in composites.
- Functional Fillers & Additives: Silica fume or bentonite adjust rheology, shrinkage, and thermal expansion, optimizing performance.
Additives and impurities play dual roles: while minor additions improve workability or thermal shock resistance, impurities like alkali oxides can weaken bonds, urging strict raw material control. MXS Refractories leverages these principles to tailor solutions for industries where failure is not an option—proving that innovation lies beyond the brick.
How chemical composition translates to performance
The performance of refractory products hinges on their chemical composition. Each element serves a specific purpose, aligning with the environmental demands of high temperatures, chemical exposure, and mechanical stress. For instance, refractories with high Al₂O₃ content excel in environments requiring exceptional refractoriness and structural integrity under load. These materials maintain stability even at temperatures exceeding 1770°C, making them ideal for steelmaking and cement kilns.
MgO-rich compositions, such as magnesia-carbon bricks, are engineered to withstand basic slags—a common challenge in steel production. Their resistance to iron-rich environments ensures longevity in metallurgical furnaces. Similarly, the inclusion of carbon (graphite) in these bricks enhances thermal shock resistance and prevents wetting by molten metals, reducing erosion and improving efficiency.
- High Alumina (Al₂O₃): Leads to superior refractoriness and load-bearing capacity.
- High Magnesia (MgO): Provides excellent resistance to basic slags and high-iron environments.
- High Silica (SiO₂): Offers great strength under load at high temperatures in acidic environments.
- Carbon (C) & Silicon Carbide (SiC): Deliver exceptional thermal shock resistance and non-wettability.
ZrO₂ (zirconia) stands out for its ultra-high service temperatures (up to 2200°C) and low thermal conductivity. Stabilized zirconia grades, like yttria-partially stabilized zirconia (Y-PSZ), resist degradation in oxidizing environments, making them critical for gas turbines and thermal barrier coatings. Their ability to maintain structural integrity under extreme thermal cycling underscores their role in demanding applications.
Additives and impurities further refine performance. Small quantities of silicon carbide (SiC) in carbon-bonded refractories amplify thermal conductivity and oxidation resistance. Conversely, impurities like alkalis in silica bricks can lower refractoriness, emphasizing the need for precise compositional control. By tailoring these elements, refractories achieve tailored performance, whether in glass furnaces, steel ladles, or petrochemical reactors.
Choosing the Right Composition for Optimal Refractory Solutions
The performance of refractory materials is inherently tied to their chemical composition. Understanding the interplay of major oxides like Al₂O₃, SiO₂, and MgO, alongside minor additives, is critical for ensuring structural integrity and efficiency in high-temperature environments. Even trace impurities from raw materials can compromise properties like thermal stability or corrosion resistance, making precise compositional analysis non-negotiable for industrial applications. This foundational knowledge directly impacts durability under extreme conditions.
Selecting the ideal refractory solution demands more than generic choices. Each application—whether in steelmaking, glass production, or cement processing—requires tailoring the chemical profile to counter specific challenges: thermal shock, abrasive wear, or chemical corrosion. For instance, silica-based products excel in acidic environments, while magnesia-carbon bricks resist basic slags. Additives like chromia or zirconia further refine properties, enabling materials to withstand harsher operational demands through controlled phase transformations or enhanced sintering.
Leveraging decades of expertise, MXS Refractories delivers customized refractory systems engineered for precision. By aligning compositional science with industry-specific challenges—such as optimizing alumina purity for steel ladels or integrating carbon matrices for thermal shock resistance—we ensure materials perform reliably. Explore our refractory glossary to decode technical terminology and deepen your understanding of material selection criteria.
The performance of refractory materials is fundamentally rooted in their composition. By balancing principal components, additives, and managing impurities, industries can tailor solutions for extreme conditions. Selecting the right composition ensures durability in high-temperature environments. For expert guidance on refractory selection, visit [MXS Refractories](https://mxs-refractories.com/) and explore our comprehensive [refractory glossary](https://mxs-refractories.com/glossary-refractories/).
