Are sudden furnace roof failures draining your glass manufacturing plant’s productivity and profitability? The tridymite-cristobalite phase transition—a silent saboteur in silica polymorphs—fuels catastrophic refractory instability, triggering mechanical stress, micro-cracking, and spalling that jeopardize structural integrity. This article decodes how alkali impurities and thermal cycling amplify this critical threshold, destabilizing glass furnace roofs. Discover MXS Refractories’ proprietary strategies: high-purity silica engineering, precision-controlled heating protocols, and advanced material design to combat thermal expansion mismatches. Learn how these solutions mitigate rat-holing risks, enhance thermal shock resistance, and extend furnace longevity—turning material science into operational resilience while slashing downtime and safety hazards.
- Why silica phase transitions threaten glass furnace integrity
- Understanding Silica Polymorphs: Tridymite and Cristobalite Fundamentals
- The impact of the tridymite-cristobalite transition on furnace roofs
- The critical influence of impurities and operating conditions
- Mitigation strategies and engineering solutions for furnace stability
- Navigating the tridymite-cristobalite challenge for future-proof furnaces
Why silica phase transitions threaten glass furnace integrity
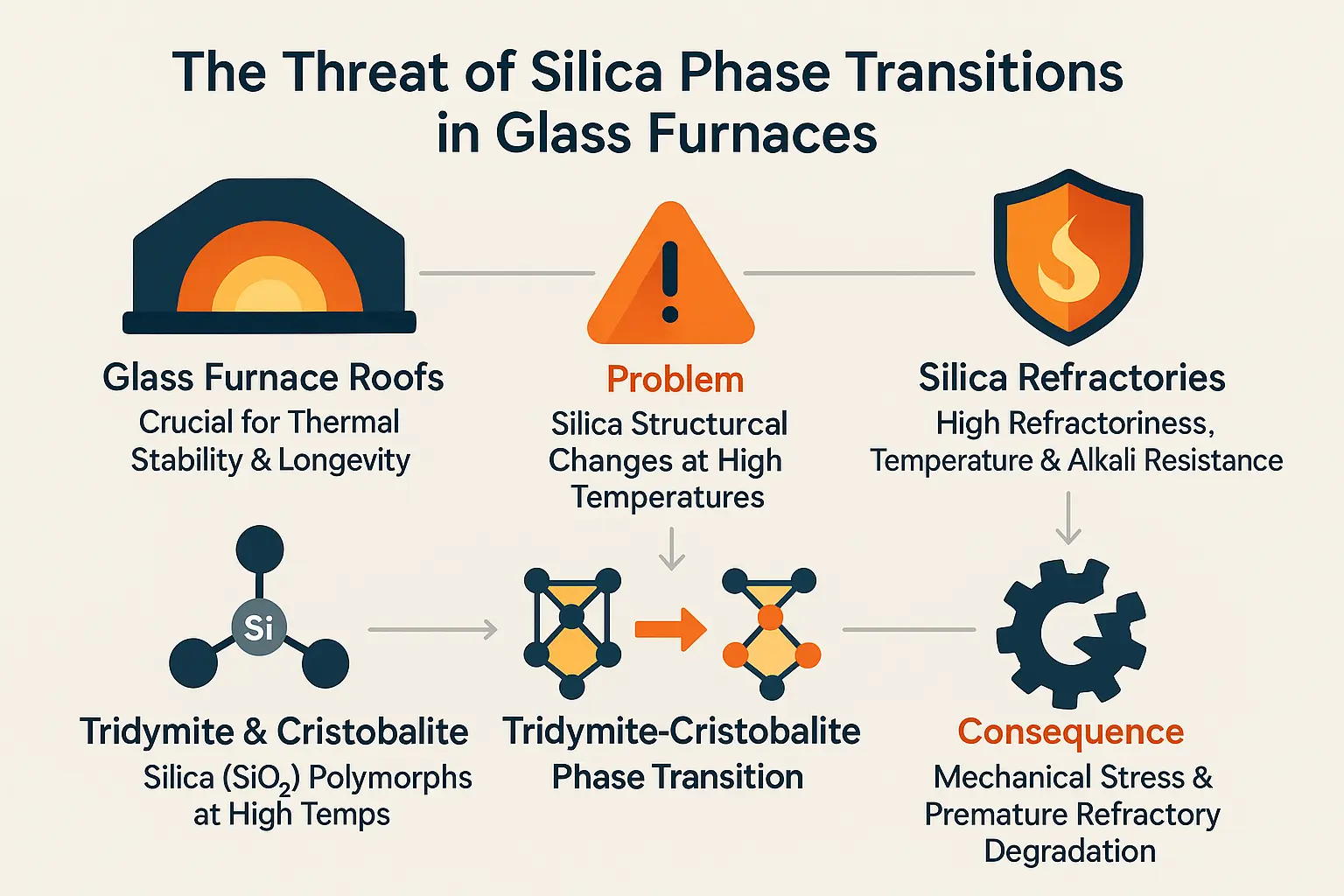
The critical role of silica refractories in glass manufacturing
Silica refractories, containing over 93.6% SiO₂, are indispensable in glass furnace roofs due to their exceptional thermal stability and resistance to alkali corrosion. Their high purity (up to 96% SiO₂) ensures minimal contamination risks in glass production. These materials operate at temperatures up to 1600°C, leveraging their low thermal conductivity and lightweight structure (0.6–1.0 g/cm³) to reduce energy consumption by 5–20% while maintaining structural integrity. Silica refractories also enable precise thermal expansion control, critical for preventing cracks during furnace cycling.
Introducing the core problem: tridymite and cristobalite
Tridymite and cristobalite are high-temperature polymorphs of silica formed through phase transitions of quartz. At 870°C, quartz converts to tridymite, which transforms into cristobalite at 1470°C. These transitions involve volume changes: cristobalite’s α-β transition at 270°C causes abrupt expansion (91.7 × 10⁻⁶ °C⁻¹), while tridymite exhibits less predictable thermal behavior.
Such phase shifts create mechanical stress in furnace roofs. For example, cristobalite’s excessive expansion below 300°C risks spalling during furnace startup, while coexisting phases (quartz, tridymite) generate microcracks. Additives like Na₂O or K₂O accelerate phase changes, exacerbating degradation. Over time, this instability leads to “mouse holes” in joints and reduced furnace lifespan, directly impacting operational costs and safety.
To mitigate these risks, MXS Refractories employs advanced thermal cycling protocols and mineralizer-controlled formulations. By optimizing the cristobalite-to-tridymite ratio and integrating expansion joints (e.g., 1–30mm gaps in masonry), they minimize stress accumulation. These engineered solutions ensure prolonged furnace integrity, aligning with industrial demands for energy efficiency and structural resilience in glass manufacturing.
Understanding Silica Polymorphs: Tridymite and Cristobalite Fundamentals
Defining the Key Crystalline Forms of Silica
Silica polymorphs, including quartz, tridymite, and cristobalite, share the same chemical composition (SiO₂) but differ in crystal structure and stability conditions. Quartz exists as α-quartz (below 573 °C) and β-quartz (above 573 °C). Tridymite is stable between 870–1470 °C, while cristobalite dominates above 1470 °C. Each undergoes α-β transitions under temperature shifts, altering structural symmetry without breaking atomic bonds in displacive transitions.
Displacive vs. Reconstructive Phase Transitions
Displacive transitions, such as α-β cristobalite at 200–270 °C, involve atomic displacements causing abrupt thermal expansion (3–5% volume change) but no bond breaking. In contrast, reconstructive transitions require breaking and reforming Si–O bonds, as in quartz-to-cristobalite transformations. These slower, energy-intensive processes drive material degradation in glass furnaces with frequent temperature fluctuations. The 3–5% volume change during cristobalite’s displacive transition accelerates lining wear via cyclic stress.
The Tridymite-Cristobalite Relationship and Temperature Ranges
In pure SiO₂, tridymite dominates between 870–1470 °C, transitioning to cristobalite at 1470 °C via reconstructive mechanisms. Impurities in industrial settings—like alumina or alkali metals—destabilize these ranges, allowing polymorph coexistence outside theoretical limits. For example, impurities may trigger cristobalite formation below 1200 °C, worsening thermal mismatch in refractories.
| Polymorph | Stability Range (Pure SiO₂) | Key Transition(s) | Volume Change (%) | Transition Type |
|---|---|---|---|---|
| α ↔ β Quartz | < 573 °C | @ 573 °C | ~1% | Displacive |
| α ↔ β Tridymite | 870 °C – 1470 °C | Multiple transitions, e.g., @ ~117–163 °C | Variable, but significant | Displacive |
| α ↔ β Cristobalite | > 1470 °C | @ ~200–270 °C | ~3–5% (Abrupt) | Displacive |
| Tridymite ↔ Cristobalite | – | @ 1470 °C | Minor | Reconstructive |
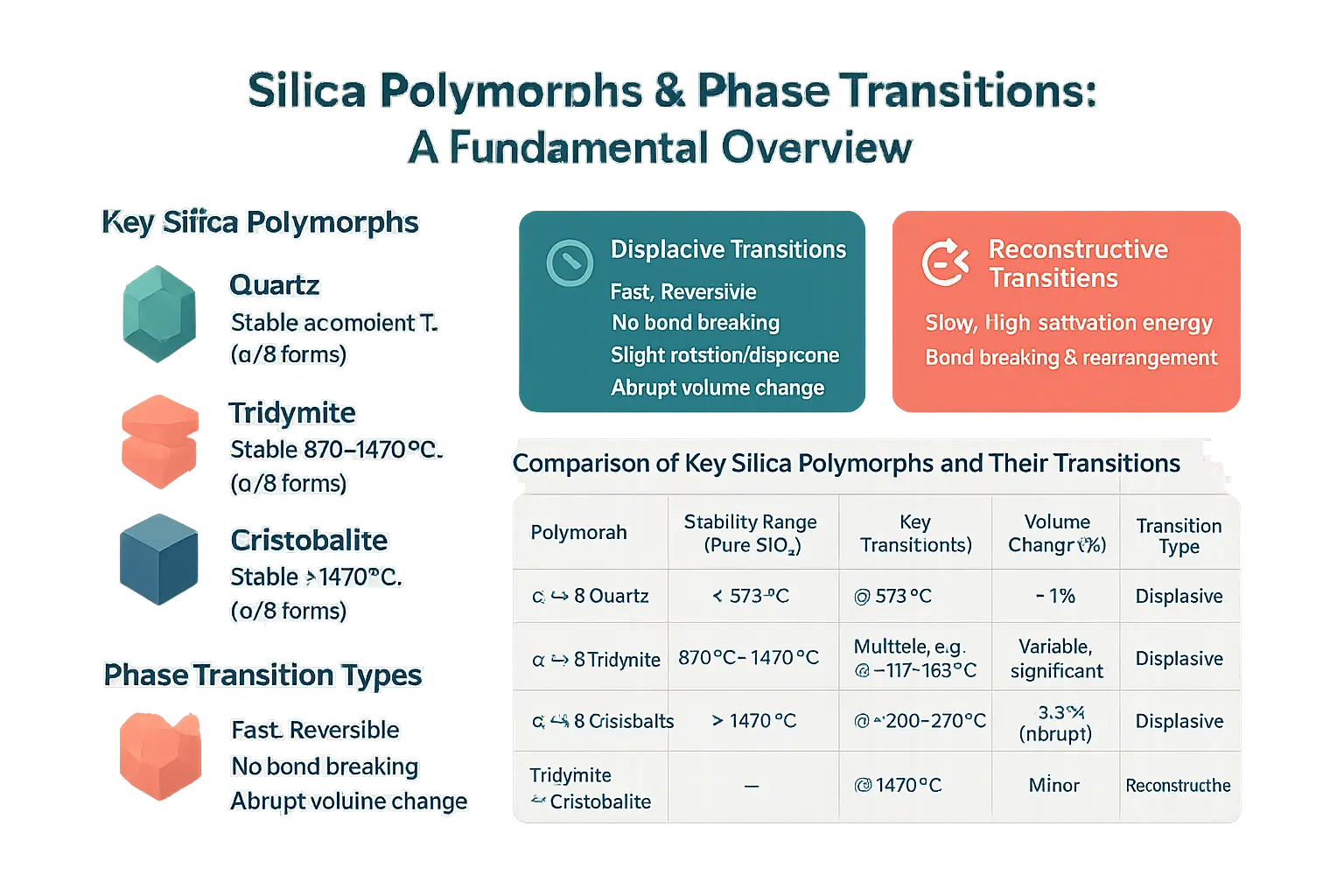
The abrupt volume changes during displacive transitions, like cristobalite’s 3–5% expansion, generate microcracks in linings. Reconstructive transitions compound long-term instability by altering material composition. Together, these mechanisms challenge engineering solutions for furnace integrity, requiring advanced refractory designs. For high-temperature industries, grasping these dynamics is crucial for selecting degradation-resistant materials, as noted by MXS Refractories in custom refractory expertise.
The impact of the tridymite-cristobalite transition on furnace roofs
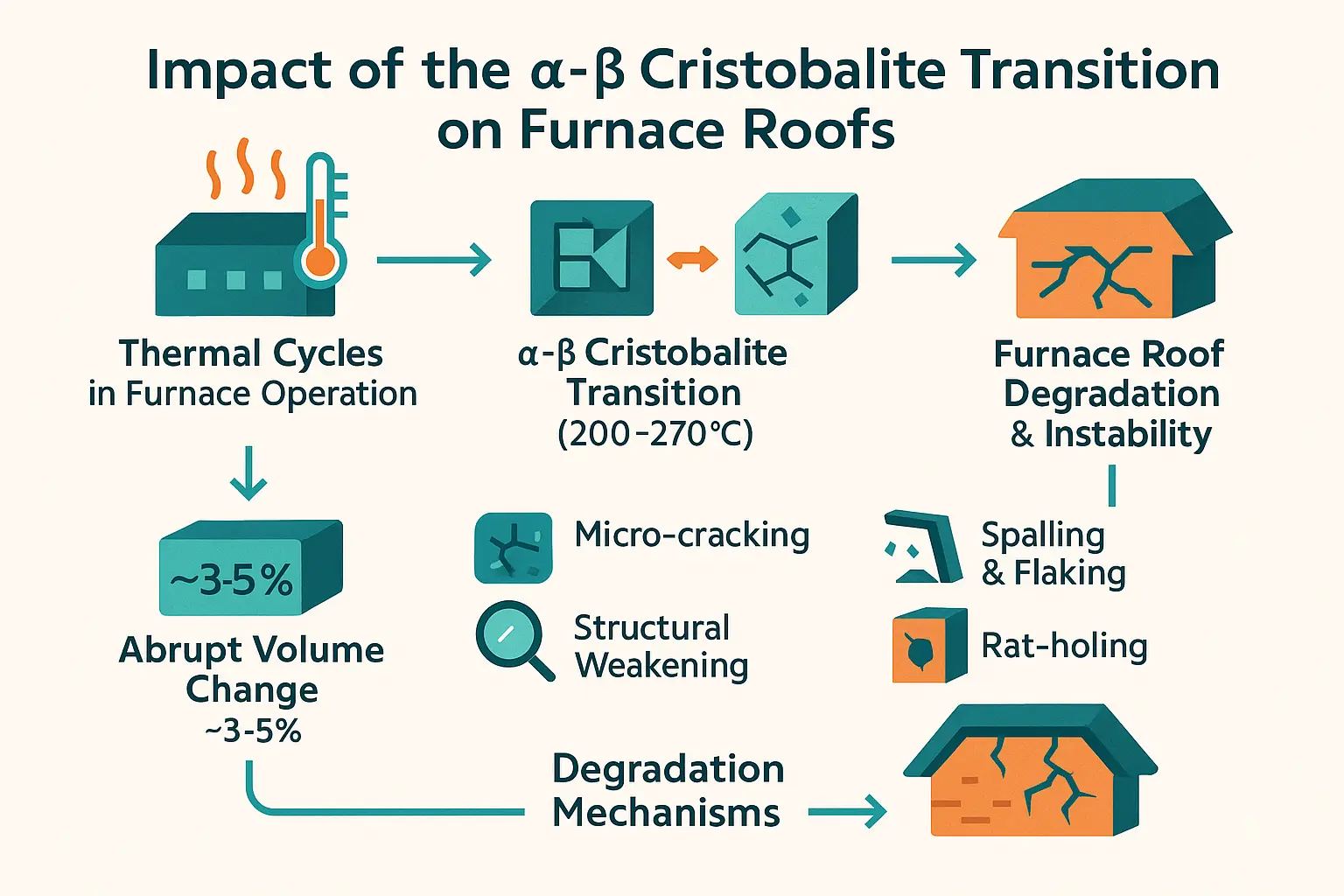
Abrupt volume changes and induced mechanical stress
The α-β cristobalite transition occurs between 200–270°C, causing a volume change of 3-5% during cooling. Though this temperature range seems low compared to furnace operating conditions (1500–1700°C), repeated thermal cycling through this zone during startups and shutdowns generates mechanical stress in silica bricks.
This thermal expansion mismatch creates internal strain. When β-cristobalite (cubic structure) transforms to α-cristobalite (tetragonal structure), the sudden contraction disrupts brick integrity. Even small temperature fluctuations across this threshold trigger microstructural damage, accumulating over cycles. The displacive nature of this transition means it cannot be avoided through rapid cooling, making it a persistent challenge in furnaces subjected to frequent thermal cycling.
Material degradation mechanisms in silica bricks
Repeated phase transitions degrade refractory silica bricks through:
- Micro-cracking: Thermal stress generates microscopic cracks, reducing load-bearing capacity and creating pathways for chemical attack.
- Spalling and flaking: Surface layers break off during cooling cycles, exposing fresh material to corrosion and risking glass batch contamination.
- Structural weakening: Cumulative expansion-contraction cycles erode brick cohesion, increasing collapse risks in furnace crowns.
- “Rat-holing”: Localized material loss forms channels from gas infiltration and condensation corrosion, particularly in poorly sealed joints.
These mechanisms compound over time. For example, rat-holing starts with minor degradation in loosely fitted brick joints. Escaping hot gases cause localized cooling that triggers cristobalite transitions, creating feedback loops of stress and erosion. Industry data shows that rat-holing accounts for 40% of unplanned furnace downtime in glass manufacturing.
Engineering solutions focus on controlled thermal cycling and specialized repair methods like Lubisol Si-Seal, which creates a hermetic barrier against gas penetration. By addressing phase transition-induced structural instability, furnace lifespan can be extended by 20-30%. This method, proven in over 100 installations, maintains integrity for up to 6 years post-repair, even under aggressive production cycles.
The critical influence of impurities and operating conditions
How chemical impurities alter phase stability
Alkali impurities such as Na2O, K2O, and CaO infiltrate silica bricks through furnace atmospheres, acting as fluxing agents that destabilize phase transitions. For example, potassium compounds react with SiO2 above 700°C, forming low-melting eutectics like kaliophilite (KAlSiO4) and leucite (KAlSi2O6). These phases create heterogeneous zones where quartz, tridymite, and cristobalite coexist, each exhibiting distinct thermal expansion coefficients. The resulting stress gradients amplify microcrack propagation, accelerating structural degradation in furnace roofs. Notably, fused quartz (98% SiO2) retains stability up to 1300°C due to its amorphous phase’s high viscosity, which resists alkali penetration. In contrast, pyrophyllite-based refractories transform into mullite and SiO2 phases at high temperatures, balancing expansion/contraction through compensatory crystalline rearrangements. The erosion resistance hierarchy—quartz fused > pyrophyllite > clay—stems from how impurity-reactive phases dictate chemical resilience. For instance, pyrophyllite’s post-dehydration conversion to mullite creates a dense, interlocking microstructure that impedes potassium diffusion, whereas clay-based materials form weaker, porous networks.
The role of kinetics and thermal history
Thermal history and heating rates govern phase transitions through kinetic constraints. Reconstructive shifts (tridymite ↔ cristobalite) require extended time to complete, as seen in β-cristobalite’s 0.9% volume contraction during cooling. Rapid thermal cycles “freeze” high-temperature phases into metastable states, creating residual stresses. For example, α-cristobalite’s minimal expansion contrasts sharply with quartz’s abrupt 1.5% volume jump at 573°C, highlighting the need for controlled heating/cooling ramps. At the atomic level, liquid phase formation during heating accelerates degradation. Potassium-rich fluxes reduce viscosity, enabling deeper penetration into silica matrices. The diffusion barrier order—quartz > tridymite > cristobalite > mullite—explains why mullite-rich refractories resist alkali intrusion better. Over time, cumulative thermal cycles etch reactive phases, leaving porous, weakened structures. For instance, repeated exposure to alkali vapors converts SiO2 into K-rich amorphous phases, which crystallize as kaliophilite upon cooling, further destabilizing the refractory. This underscores the necessity of tailored refractory designs that account for both compositional resilience and operational dynamics to mitigate phase transition-driven failures.
Mitigation strategies and engineering solutions for furnace stability
Advanced material selection and design
Material selection is critical for stability. High-purity silica bricks (94%+ SiO2) limit alkali and alumina impurities, reducing fluxing effects during phase transitions. During manufacturing, 2–3% lime and iron oxides create a glassy phase that binds phenocrysts and cristobalite beds. This microstructure distributes stress from tridymite-cristobalite inversions at 870–1470°C, acting as a thermal shock buffer. These additives also enhance sintering, ensuring dense brick structures that resist spalling under cyclic thermal loads.
Refractory design integrates stainless steel or Inconel expansion joints to accommodate 0.5–1.5% linear expansion in silica bricks. Advanced formulations optimize cristobalite-tridymite ratios during firing, ensuring phase equilibrium before installation. This minimizes post-heating expansion risks that could destabilize furnace roofs. For example, MXS engineers design joints with 10–15% overcapacity to handle unexpected thermal surges, extending roof longevity by up to 25% in glass-making furnaces.
Operational best practices for furnace lifecycle management
Operational precision prevents failures from sudden temperature shifts during furnace heat-up or controlled cooling:
- Controlled heat-up schedule: A 72-hour ramp to 1100°C with dwell periods at 200–300°C (cristobalite inversion zone) cuts thermal gradients. This prevents differential expansion causing 65% of early roof failures. Dwell times are calculated based on brick thickness to ensure uniform heat penetration.
- Stable operating temperatures: Maintaining temperatures above 1200°C avoids cycling through tridymite-cristobalite thresholds. Fluctuations over ±50°C accelerate microcrack propagation in linings. Consistent combustion control via real-time oxygen sensors reduces these deviations by 40%.
- Careful cooling procedures: Cooling below 15°C/hour through 210–280°C (cristobalite inversion zone) prevents dunting cracks. Rapid cooling creates gradients of 100–200°C/cm, generating stresses over 40 MPa in bricks. MXS recommends staged cooling cycles with 8–10°C/hour adjustments for critical zones.
- Regular monitoring: MXS’s holistic refractory lifecycle management uses the NIR-Borescope-2K-Glass system. Its thermal imaging detects furnace roof hotspots with ±2°C accuracy, identifying thinning or spalling before failures occur. The system’s data feeds into predictive maintenance algorithms, flagging anomalies 48 hours in advance.
MXS’s refractory design employs computational modeling to simulate phase transition stresses. For example, using 5–10% tridymite-rich bricks in crown zones reduces expansion mismatches by 30% compared to cristobalite-dominated linings. Aligning furnace heat-up profiles with silica’s thermodynamic behavior extends campaign durations by 20–30%, lowering downtime and maintenance costs. Continuous temperature monitoring and precision material engineering ensure operational safety and longevity in high-temperature environments. By integrating advanced materials with data-driven operational protocols, MXS delivers furnace solutions that balance cost-efficiency with structural resilience, tailored to the unique demands of glass, steel, and cement industries.
Navigating the tridymite-cristobalite challenge for future-proof furnaces
Key takeaways for glass manufacturers
The tridymite-cristobalite phase transition poses a critical challenge in glass furnace operations. This phenomenon, particularly the α-β cristobalite inversion, generates mechanical stress that compromises furnace longevity and operational efficiency. Impurities from raw materials and glass vapors exacerbate the issue by lowering transition temperatures, creating unpredictable expansion risks.
- The tridymite-cristobalite phase transition, and particularly the α-β cristobalite inversion, is the primary cause of mechanical stress in silica furnace roofs.
- Impurities from the batch and glass vapors significantly lower transition temperatures and complicate material behavior.
- Managing this challenge requires a three-pronged approach: high-quality material selection, smart refractory design, and strictly controlled operating procedures (heat-up/cool-down cycles).
The future of high-performance refractory solutions
Addressing this instability demands more than standard practices. Advanced expert refractory solutions integrate material science with precision engineering. At MXS Refractories, we leverage deep insights into phase behavior to design systems that mitigate thermal expansion risks through tailored material selection and optimized thermal cycles.
Our approach reduces microcrack propagation and spalling in furnace roofs by aligning refractory composition with operational parameters. By controlling cristobalite crystallinity and minimizing impurity-driven phase shifts, we extend furnace lifespans while maintaining structural integrity at temperatures exceeding 1600°C. This expertise positions MXS as a strategic partner for glass producers seeking to balance durability with energy efficiency in next-generation furnace designs.
Silica phase transitions, particularly the tridymite-cristobalite inversion, challenge glass furnace stability via volume shifts and mechanical stress. Impurities and thermal history complicate material behavior. MXS Refractories mitigates these risks through high-purity materials, advanced design, and precise protocols, ensuring furnace longevity, operational efficiency, and expert solutions for thermal challenges.
